Some of these are listed below. Chemistry questions and answers.
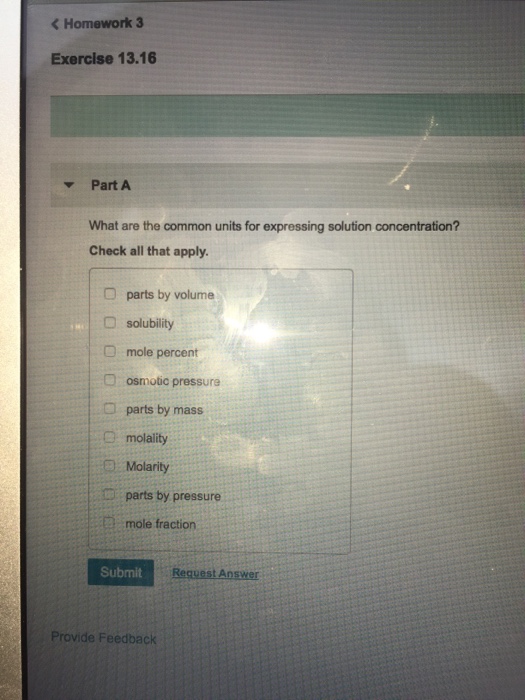
Solved Omewo Exercise 13 16 Part A What Are The Common Units Chegg Com
Molarity M indicates the number of moles of solute per liter of solution molesLiter and is one of the most common units used to measure the concentration of a solution.

. Which unit can be used to express the concentration of a solution. The concentration of the solution formula is given as follows. Thus if 20 grams of table salt is dissolved in 80 grams of water the concentration of this solution in mass percent will be.
If water is the solvent the solution is called an aqueous solution. Concentration of solution. It is called 10 solution.
A solution can be qualitatively described as. We will also see other methods on how to calculate the concentration of a solution based on the different methods of expressing concentrations. Molarity can be used to calculate the volume of solvent or the amount of solute.
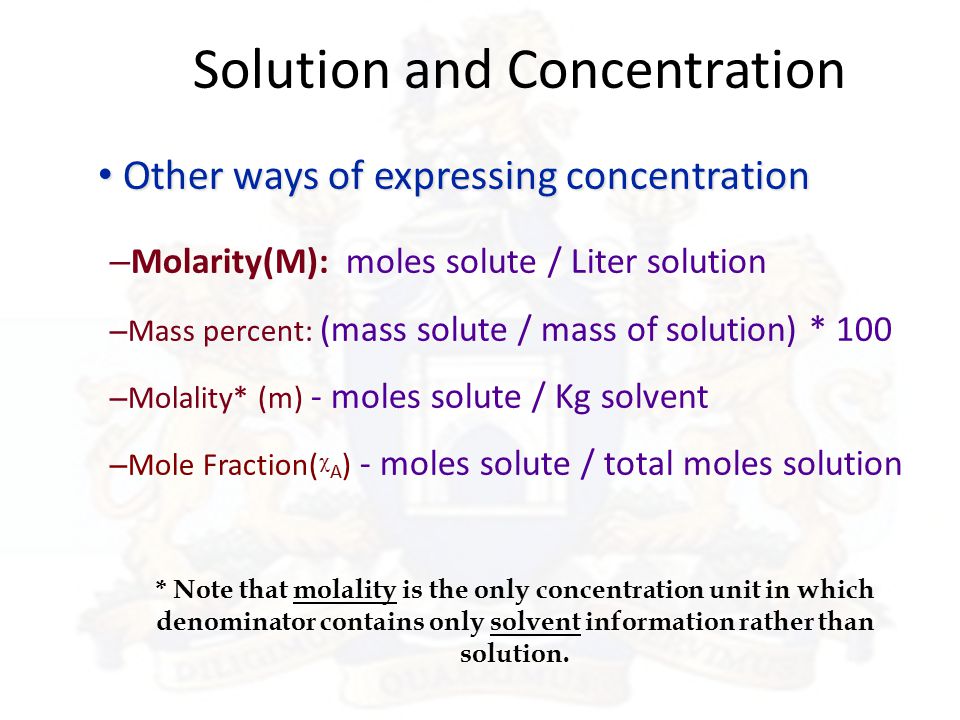
Although units of weight percent and mole fraction can be applied to all types of solutions the most common concentration terms are molarity or molality. When the concentration is expressed as the percent of one component in the solution by mass it is called mass percentage ww. What are the common units for expressing solution concentration.
So the unit of this term is gram per liter. It is also independent of the variation in temperature. Check all that apply.
Parts by mass h. Molarity can be used to calculate the volume of solvent or the amount of solute. Chemistry questions and answers.
Weight of the solute in gram volume in Litres. One mole of any substance is equal to its molecular weight. What are the common units for expressing solution concentration.
The molarity is the number of moles or gram formula masses of solute in 1 liter of solution. Concentrations can also be show through mass per univ volume. Lastly it can also be expressed through percent by mass or volume.
PPM M x M. It can be expressed by the following formula. Parts by volume g.
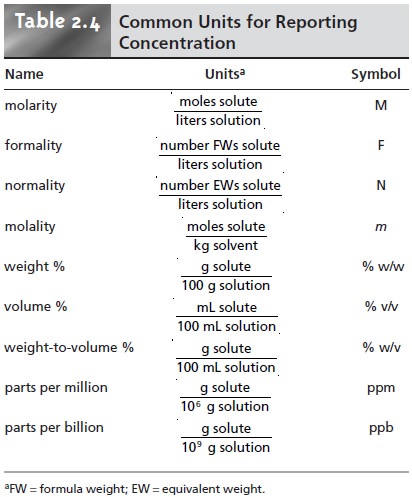
Check all that apply parts by volume solubility mole percent osmotic pressure parts by mass molality a Molarity parts by pressure mole fraction Submit Request Answer Provide Feedback. Molarity and molality is widely used as units for expressing concentration. Molarity M moles soluteliter of solution Normality N equivalents of soluteliter of solution Weight Wt mass of solutemass of solution x 100 Parts per million ppm mass of solutemass of solution x 106.
Strength or Solution Concentration Concentration in Gram per Liter. The number of moles of a solute per liter of a solution is called molarity. There are a number of different ways of expressing solute concentration that are commonly used.
Qualitative Expressions of Concentration. So the first and probably the most common way of expressing the concentration is similarity which can be also looking simply as. Another example is by parts such as parts per million and parts per billion.
The unit of molarity is mol kg-1. Molarity of any solution is number of moles of solute per liter of solution. The molarity of a solution is the number of moles of the solute contained in one liter of the solution.
Concentration in Parts per Million. Another common way of describing concentration is as the. Presence of a Nonvolatile Solute in a Liquid Results in.
This is a common way to express the concentration of a solution in which the solute is a solid substance. Concentration in Parts Per Million ppm The parts of a component per million parts 10 6 of the solution. Molarity Number of moles of solute Volume of solution in liter.
Molarity M Molality m mole fraction x mole percent mol percent by mass or volume parts per million ppm by mass or volume and parts per billion ppb by mass or volume. The volume or weight concentration of some solute is expressed in the percentage. And it is defined as the amount off so moot in wall divided by the volume off the solution in leaders said these.
Concentration of solution Solute mass in gram Solution volume in liters. Calculate the molar concentration of 2000 ppm of Pb 2 A. Find step-by-step Chemistry solutions and your answer to the following textbook question.
Mass percent refers to the weight of the solute in grams per 100 g of the solution and not the solvent. Ways of expressing concentrations of solutions in chemical units. Thus if one gram molecule of a solute is present in 1 kg of the solvent the concentration of solutions is said to be one molal.
OmewO Exercise 1316 Part A What are the common units for expressing solution concentration. Molality is the most convenient method to express the concentration of solutions because it involves the mass of liquids rather than their volumes. The concentration of a solution is a macroscopic property represents the amount of solute dissolved in a unit amount of solvent or of solution and can be expressed in a variety of ways qualitatively and quantitatively.
Common Units Used to Express Solution Concentration. Thus because the density of water under standard conditions is very close to 10 gmL the volume of 10 kg of H_2O under these conditions is very close to 10 L and a 050 M solution of KBr in water for example has approximately the same concentration as a 050 m solution. 20 divided by 100.
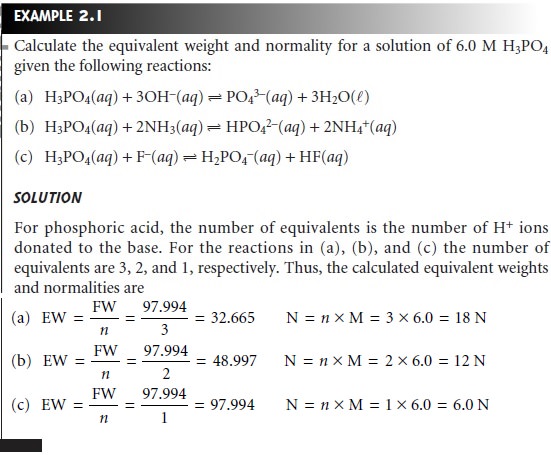
Molarity M indicates the number of moles of solute per liter of solution molesLiter and is one of the most common units used to measure the concentration of a solution. The formality of a solution is the number of gram-formula weights of the solute contained in one liter of the solution. For example 10 gin of sodium chloride is added in 90 gm of water.
This term can be defined as the amount of the solute mass in gram present in one liter of the solution.
0 Comments